How Many Particles Are in 1 Mole of Carbon
So a mole of water H2O has a mass of 18 g. List the conversion factors used to convert between particles and moles.
Chemical Quantities The Mole Ppt Download
Of atoms in it is equal to.

. Carbon monoxide CO has one atom. One mole contains 602 x1023 representative particles. That is why it is given a name.
Of atoms in 125 moles of carbon 6022 x 1023 x 125 753 x 1023 answer 98 views View upvotes. How many molecules of carbon dioxide have a mass of 720 g. This would mean that all of the items described would have approximately 6022 1023.
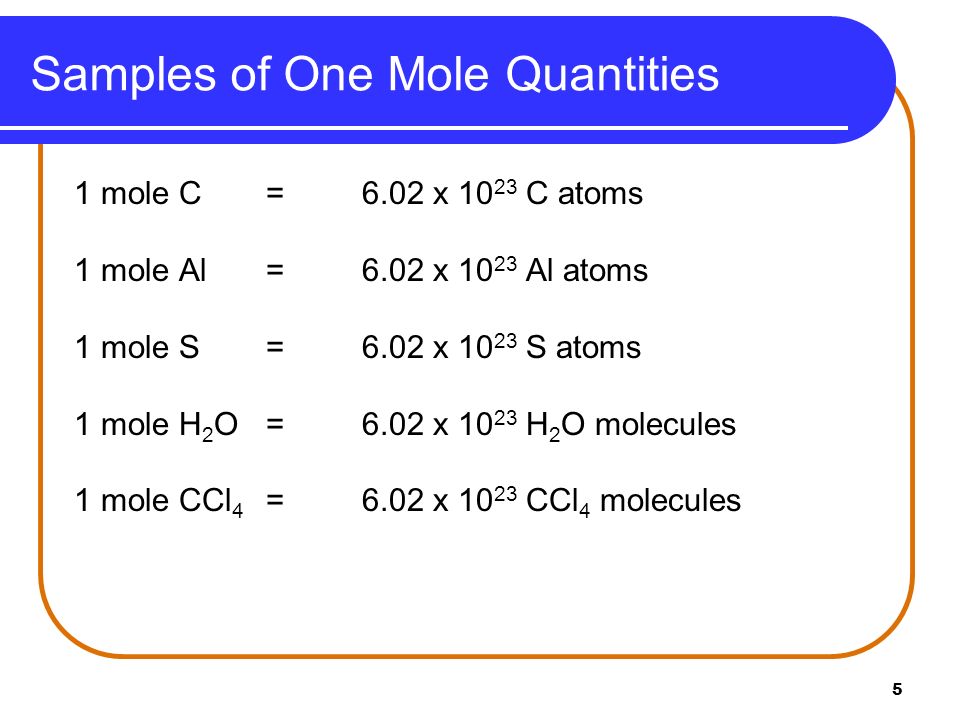
Hence the number of atoms in 01 m o l of carbon atom is 01 N A atoms or 6022 10 22 atoms. 1 mole of carbon contains 6022 x 1023 Avogadro number atoms of carbon. 1200 g C-12 1 mol C-12.
Up to 24 cash back One mole of carbon still has 6022 10 23 carbon atoms but 9889 of those atoms are carbon-12 111 are carbon-13 and a trace about 1 atom in 1012 are. A mole of a molecular compound contains 6 x 1023 molecules. Type in answers without units using scientific notation - include three decimal places.
The number of atoms or other particles in a mole is the same for all substances. The number of atoms molecules or ions in one mole of a substance is called the Avogadro constant. As we know that one mole of carbon weighs 12g the total no.
Therefore 12 g of C-12 contains 12 1992648 10 23 60221367 10 23 6022 10 23 atoms. One mole of a substance contains Avogadros number of atomsmoleculesthe like. Exactly 12 grams of pure carbon-12 powder is.
Solution for How many particles are contained in 543 moles of carbon. How many carbon atoms are in 100 mole of C2H6. One mole of carbon-12 atoms has.
The mole is related to the mass of an element in the following way. 1- 1 Mole of C 12 grams 6023 X 10 23atoms01 Moles of C 01 X 6023 X 10 23atoms 6023 X 10 22atomsTherefore 01 moles of Carbon contain 6023 X 10 22atoms2-Molecular. It has a mass that is equal to its relative formula mass.
1 m o l e. Mole calculations are just like converting from inches to feet or. The mole is defined as exactly 6022 140 76 1023 elementary entities.
Its value is 602 1023 per mole which is. Of entities in 1 mole plays a vital role in calculations in chemistry. In short 1 mole 6021023 particles 481023 particles 6021023 particlesmol 0797 mol What is the molar mass of carbon monoxide.
1 mole of any substance 6023 1023 particles atomsionsmolecules So 1 mole of CO2 will have 6023 x1023 molecules Cheers. Number of mol is calculated by ratio of given mass to the molar mass. Up to 24 cash back MOLES PARTICLES To convert between moles and particles you must always use Avogadros number 602 1023 particles mol.
Thus there are 6022 10 23 atoms in exactly one. C 361 x 1024. A 301 x 1023.
Explain how a mole is similar to a dozen. 21K views View upvotes Mohammad Sayed Immam. October 31 2021 thanh.
26 KB Referencing Hub media. A mole of carbon dioxide. Avogadros number is 602 x 1023 particles.
The amount of material counting 602214 1023 particles The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. What is the mass of 75 moles of carbon dioxide 440 g1 mole. This definition was adopted in November 2018 and came into force on the 20 May 2019 superseding the previous.
One mole of C-12 weighs 12g. Andrew Lambert PhotographyScience Photo Library Published 17 August 2011 Size. The particles can be atoms molecules or ions.
One mole is defined as the amount of substance that contains as many particles as the number of atoms in exactly 12 g of carbon-12 which is 602 10 23 particles.

Stoichiometry And The Mole Test Unit Exam Exam Mole Concept The Unit
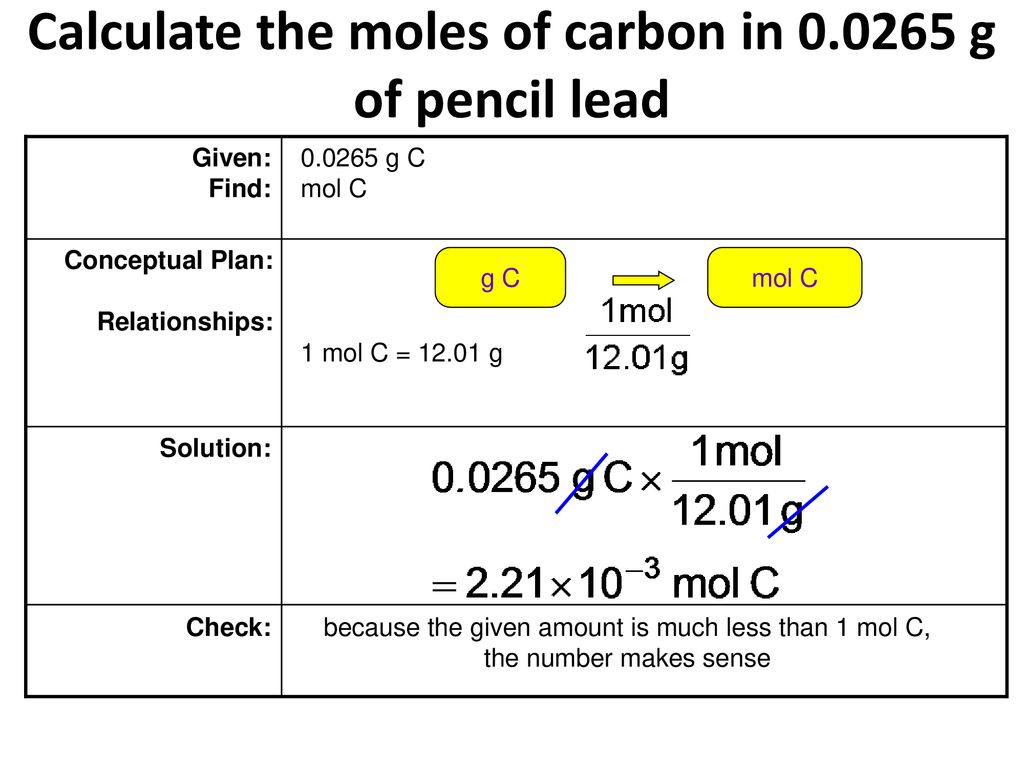
Calculate The Moles Of Carbon In G Of Pencil Lead Ppt Download

If One Mole Of Carbon Atoms Weighs 12 Grams What Is The Mass In Grams Of 1 Atom Of Carbon Youtube
How Many Atoms Of Carbon Are Present In 0 1 Mole Of C12h22o11 Quora
No comments for "How Many Particles Are in 1 Mole of Carbon"
Post a Comment